Which Term Best Describes All Atoms in Ionic Bonds
Which term best describes all atoms in ionic bonds. Chemical bonds are forces that hold atoms together to make compounds or molecules.
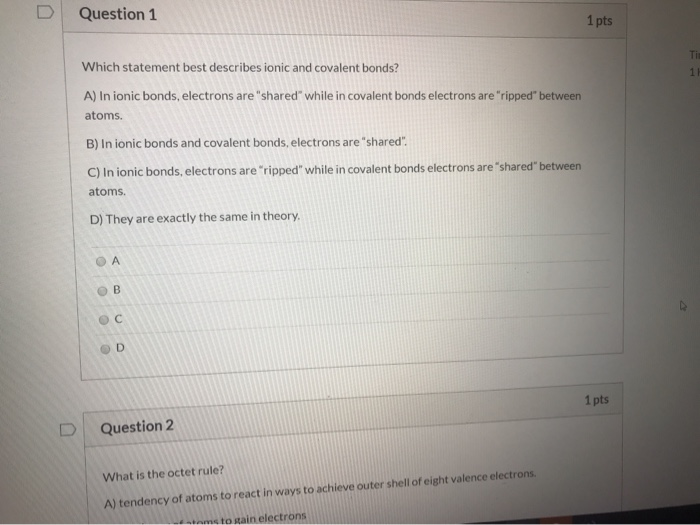
Solved Question 1 1 Pts Which Statement Best Describes Ionic Chegg Com
This exchange results in a more stable noble gas electronic configuration for both atoms involved.

. The atoms valence electrons are shared between the atoms. O 0 1 pts Question 2 What is the octet. B In ionic bonds and covalent bonds electrons are shared.
Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. A In ionic bonds electrons are shared while in covalent bonds electrons are ripped between atoms. Which term best describes all atoms in ionic bonds-stable-unstable-positively charged-negatively charged.
Atoms with large differences in electronegativity transfer electrons to form ions. She said that this demonstrates that ionic compounds have strong bonds because a lot of energy is needed to break the electrical forces that hold the bonds together. The covalent bond is a bond formed when two atoms share one or more electron pairs.
All of the atoms electrons are shared between the atoms. The ionic bond is the attraction between positive and negative ions in a crystal and compounds held together by ionic bonds are called ionic compounds. Which statement best describes the energy change as bonds are formed into this reaction.
Each atom contributes an equal number of electrons towards the bond formation. A force that holds atoms together is a _____ bond. Valence electrons are transferred from one atom to the other.
But be aware that ionic lattices also contain more than 2 atoms that are joined by ionic bonds. Atoms with relatively similar electronegativities share electrons between them and are connected by covalent bonds. The forming of the H-Cl bond releases energy.
C In ionic bonds electrons are ripped while in covalent bonds electrons are shared between atoms D They are exactly the same in theory. See answer 1 Best Answer. As the atoms of the iodine react to form molecules of iodine the stability of iodine.
Anna stated that ionic compounds have high melting points and low boiling points. Which statement correctly describes a covalent bond. An existing theory is modified so that it can explain both the old and new observations.
Chemical bonds include covalent polar covalent and ionic bonds. These lattices are not called molecules. An ionic bond is based on attractive electrostatic forces between two ions of opposite charge.
Three idealized types of bonding are ionic bonding in which positively and negatively charged ions are held together by electrostatic forces covalent bonding in which electron pairs are shared between atoms and metallic bonding. Chemical bonding is the general term used to describe the forces that hold atoms together in molecules and ions. The atoms valence electrons combine to form a network of bonds.
As a chemical bond forms between two hydrogen atoms in a system energy is released and the stability of the system. Which statement best describes how an existing theory is often affected by the development of new technology. Most isotopes of copper are very unstable and decay within seconds.

Which Of These Best Describes An Ionic Bond Brainly Com

Question 6 Of 10 Look At The Picture Below Which Best Describes The Bond Shown A A Covalent Bond Brainly Com
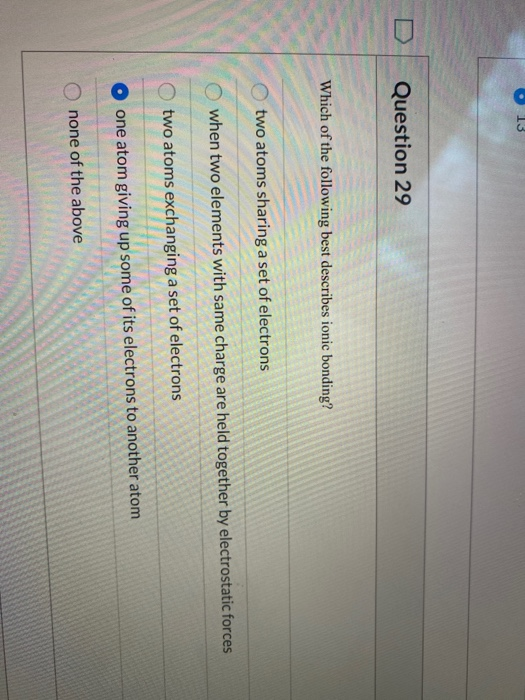
Solved Question 29 Which Of The Following Best Describes Chegg Com

Question Video Selecting The Statement That Does Not Describe Ionic Bonding Nagwa
No comments for "Which Term Best Describes All Atoms in Ionic Bonds"
Post a Comment